Chlor Alkali Plant
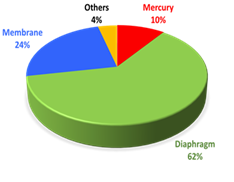 The chlor-alkali (also called “chlorine-caustic”) industry is one of the largest electrochemical technologies in the world. It is an energy intensive process and is the second largest consumer of electricity (2400 billion kWh) among electrolytic industries. In 2006, about 84% of the total world chlorine capacity of about 59 million metric tons was produced electrolytically using diaphragm and membrane cells, while about 13% was made using mercury cells.
The chlor-alkali (also called “chlorine-caustic”) industry is one of the largest electrochemical technologies in the world. It is an energy intensive process and is the second largest consumer of electricity (2400 billion kWh) among electrolytic industries. In 2006, about 84% of the total world chlorine capacity of about 59 million metric tons was produced electrolytically using diaphragm and membrane cells, while about 13% was made using mercury cells.
Chlorine is produced by the electrolysis of sodium chloride (common table salt) solution, often called “brine.” Thus, when sodium chloride is dissolved in water, it dissociates into sodium cations and chloride anions.
The chloride ions are oxidized at the anode to form chlorine gas, water molecules are reduced at the cathode to form hydroxyl anions and hydrogen gas. The sodium ions in the solution and the hydroxyl ions produced at the cathode constitute the components of sodium hydroxide formed during the electrolysis of sodium chloride.
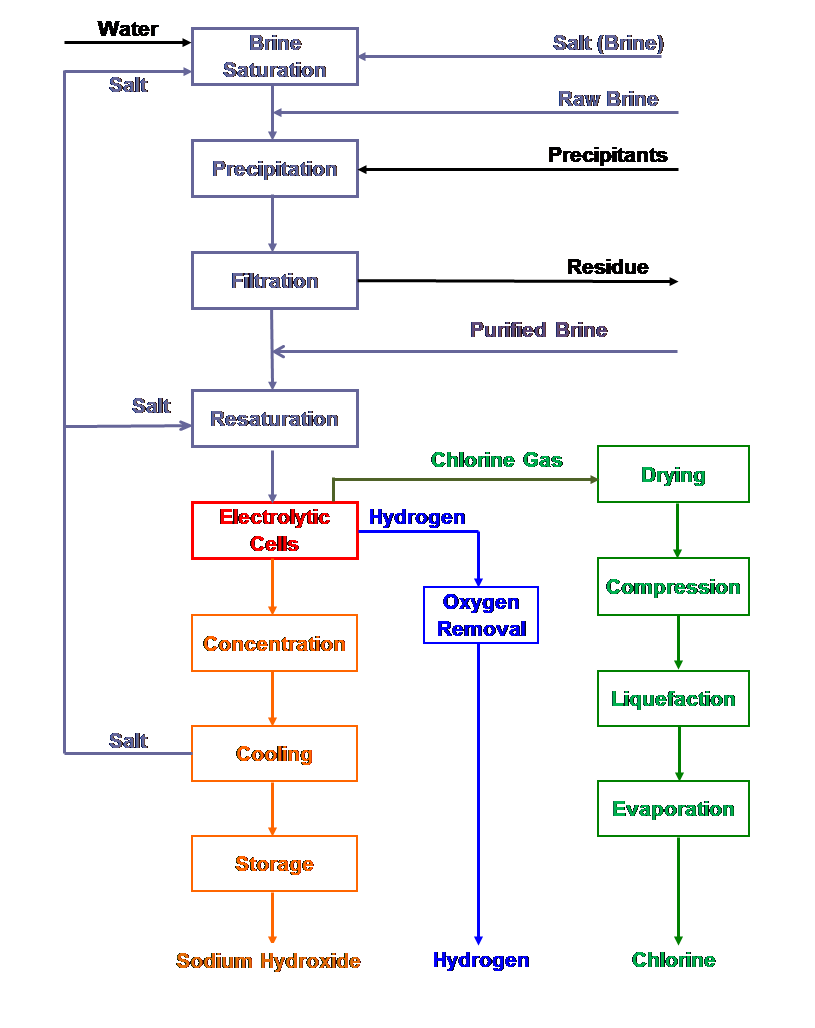
There are 3 types of electrolytic cells to produce chlorine. The main difference in these 3 technologies lies in the manner by which the chlorine gas and the sodium hydroxide is prevented from mixing with each other to ensure the purity of the products. Thus, in diaphragm cells, brine from the anode compartment flows through the separator to the cathode compartment, the separator material being either asbestos or polymer-modified asbestos composite deposited on a for aminous cathode.
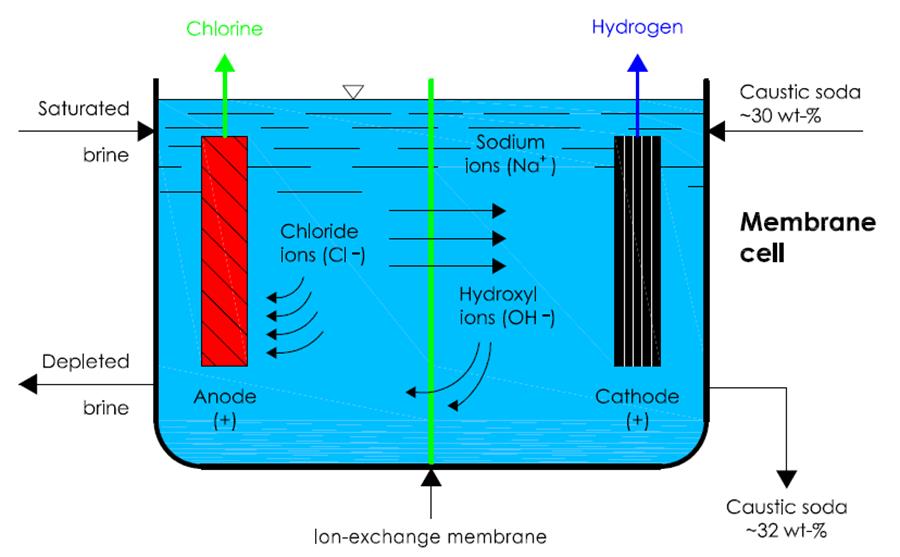
On the other hand, there is an ion-exchange membrane in membrane cells is used as a separator. Anolyte-catholyte separation is achieved in the diaphragm and membrane cells using separators and ion-exchange membranes, respectively, whereas mercury cells contain no diaphragm or membrane and the mercury itself acts as a separator. The anode in all technologies is titanium metal coated with an electrocatalytic layer of mixed oxides.
A membrane cell is a diaphragm cell with an improved diaphragm called a ‘membrane’. This is made from polytetrafluoroethylene (PTFE), making it a plastic membrane, which has been modified to include anionic groups to act as an ion exchange membrane. This allows sodium ions to pass through it but not chloride or hydroxide ions. PTFE is very inert and so can stand immersion in hydroxide solutions for long periods of time. Therefore, the same reactions occur as above in the diaphragm cell but there is no by-product of sodium chloride due to the membrane preventing the chloride and hydroxide ions to pass through.
The use of this process results in virtually pure sodium hydroxide (only contains about 0.02% of sodium chloride at most) being produced as there is no contamination by chloride, and there is virtually no oxidant in the spent brine. Furthermore, there is no working hazard of working with asbestos or mercury.
This process of using electrolysis to develop sodium hydroxide, has been adapted in all chlor-alkali plants due to its very pure production of sodium hydroxide, similar costs to that of the diaphragm cell (reasonable) and it has no negligible environmental impact. The advantages are much preferable to that of the mercury and diaphragm process and the membrane process have negligible disadvantages proving to be the best technique for extracting sodium hydroxide.
Synopsis Chemitech developed the process based on membrane electrolysis as the caustic soda plant has been adapted in all Chlor-alkali industry.